Scaling up Accessible and Effective HCV Treatment Through a Community-Based Treatment Model for Most Vulnerable Populations in the Resource- Constrained Ukraine
Scaling up Accessible and Effective HCV Treatment Through a Community-Based Treatment Model for Most Vulnerable Populations in Resource-Constrained Ukraine
Project duration: April 2015–October 2016.
Overall project aim: providing access to HCV treatment for most vulnerable populations and developing community-based innovative models of services delivery, including medical and social support of treatment with direct-acting antivirals, in particular sofosbuvir.
Project geography:
- stage 1: eight healthcare facilities in seven regions, 250 treatment courses
- stage 2: 17 healthcare facilities in 14 regions, 689 treatment courses
- stage 3: to be defined, with possible extension to 25 regions and coverage of up to 1,500 courses
Key objectives:
- Implementing a community-based HCV treatment model with the use of sofosbuvir for most vulnerable populations (PWID, SW, MSM) and for HIV-positive members of most vulnerable populations (PWID, SW, MSM)
- Ensuring support and access to laboratory diagnostics to ensure treatment monitoring and further follow-up
- Conducting research to define the most effective model of sofosbuvir-based HCV treatment for most vulnerable populations
- Integrating sofosbuvir-based treatment regimens into the National HCV Treatment Guidelines
Key achievements:
- As of 5 October 2015, a total of 214 patients were receiving HCV treatment
- 196 of them are receiving ART, including 15 people who receive ART and OST together with HCV treatment
- 78 people have already completed treatment, with 133 continuing treatment
PROJECT NEWS
Innovative Treatment of Viral Hepatitis C Proves to be Successful!

09.08.2016
Alliance for Public Health (Alliance) reports on the continuation of successful implementation of the first pilot project to treat chronic viral hepatitis C (HCV) in Ukraine with highly-effective direct-acting antivirals. While drugs, procured for state funds for 2015 are still delivered to the regions, Alliance managed to achieve the highest coverage of vulnerable populations with treatment in the country and to prove high efficiency of such treatment!
As of July 1, 2016, 1092 patients from vulnerable populations had access to HCV treatment, and 595 of them have already successfully completed their treatment courses. Nineteen healthcare facilities in 17 oblasts of Ukraine joined the project. Among patients, who underwent follow-up tests for HCV 12 weeks after the end of treatment, in 93% of people the virus was not detected. This demonstrates twice higher treatment effectiveness as compared to the traditional two-component regimen with pegylated interferon and ribavirin, which is still used in most healthcare facilities of Ukraine. Such results were achieved not only due to the introduction of an innovative drug, but also thanks to the new qualified approach to prescribing new treatment regimens, effective support from personnel of healthcare facilities, taking part in the project, and proper social support of each project participant, which is ensured by partner non-governmental organizations.
At all stages of implementation of its innovative project of chronic viral hepatitis C treatment with the use of a highly-effective direct-acting antiviral – sofosbuvir – Alliance aimed at making such treatment a standard in Ukraine and providing access to it to all patients who need such treatment, not only vulnerable populations.
Having commenced HCV treatment with sofosbuvir, which at that point was not yet registered in Ukraine, Alliance started active discussions on the need to update current clinical guidelines, which contained recommendations on more lengthy (for one year) and less effective regimen using pegylated interferon. As a result, in 2015 the National Guidelines on hepatitis C treatment was updated for the first time and on July 18, 2016 MoH approved updated Unified Clinical Protocol and Adapted Clinical Guidelines “Viral hepatitis C”. New, up-to-date treatment regimens using combinations of highly-effective direct-acting antivirals were added to the List of drugs recommended to be prescribed to all patients, including sofosbuvir, sofosbuvir/ledipasvir, simeprevir, ombitasvir/paritaprevir/ritonavir etc.
Partnership and well-organized cooperation with the Ministry of Health and state healthcare institutions allowed to engage the resources of the National and local hepatitis treatment programs, namely to use pegylated interferon procured within the national and local budgets. More than half of the patients, involved into the Alliance program, received HCV treatment using a combined regimen, including sofosbuvir, pegylated interferon and ribavirin. The duration of such treatment is only 3 months as compared to the old regimen, which took at least one year. This approach allowed increasing the planned number of patients by 50%!
In Rivne, Kyiv, and Kharkiv oblasts as well as in the clinic of Institute named after Hromashevsky all pegylated interferon, which was used in treatment regimens combined with sofosbuvir provided by Alliance, was procured exclusively with funds of the national and local budgets. The experience of joint use of resources in Ivano-Frankivsk, Sumy, and Dnipropetrovsk oblasts also proved to be rather successful.
Alliance presented the key achievements of the pilot sofosbuvir-based HCV treatment project at the international conferences and professional community meetings, where discussions were held both on the clinical aspects of treatment and scaling up access of people to highly-effective drugs to treat hepatitis C.
Currently Alliance is getting prepared to Phase 3 of the project implementation, which stipulates enrollment of at least 750 more patients on treatment and expanding the geographical coverage to all regions of Ukraine. In addition to this, introduction of new treatment regimens using highly-effective direct-acting antivirals is planned.
List of healthcare facilities, involved in Alliance HCV treatment project, could be found at link.
Additionally, any questions on HCV can be addressed to Viral Hepatitis Hotline at 0-800-50-33-10.
WHO updated its Hepatitis C treatment Guidelines

27/04/2016
In April 2016, the World Health Organization issued the updated Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection.
The first version of the Guidelines was published in 2014. Since then, new direct acting antivirals (DAAs) have been introduced, allowing to improve cure rates up to 90% or higher, minimize side effects and shorten the duration of treatment.
In the updated version of the Guidelines, the peginterferon and ribavirin regimens were replaced with regimens using new-generation DAAs (taking into account specifics of certain patient groups). Telaprevir and boceprevir, as well as their combinations with peginterferon and ribavirin, are no longer recommended for the treatment of chronic hepatitis C.
Ludmila Maistat, Senior Programme Manager: Hepatitis/HIV Policy and Advocacy in Alliance for Public Health, who is a member of the WHO Strategic and Technical Advisory Committee for Viral Hepatitis, participated in the development of 2014 Guidelines as well as their updated version.
It is expected that the Guidelines will be regularly updated with the introduction of new drugs and regimens.
You may find the policy brief here.
International Liver Congress 2016 is the annual EASL meeting

14/04/2016
On 13-17 April 2016, Barcelona hosts an annual meeting of the European Association for the Study of Liver (EASL) – International Liver Congress, ILC 2016.
Every year, this Congress is attended by over 10,000 participants: doctors, hepatologists, scientists, representatives of pharmaceutical companies, microbiologists, and government officials of the member states. This event is a great opportunity to share professional achievements and develop effective strategies.
Ludmila Maistat, Senior Program Manager: Hepatitis, Alliance for Public Health presented the results of the effective work to expand access to HCV treatment in Ukraine and in EECA region. Significant updates and important comments to the presentation on the access to treatment were made by Professor Olga Holubovska, the main independent infectious disease doctor of the Ministry of Health of Ukraine, head of the infectious disease department of the Bogomolets National Medical University.
Tetyana Barnard, Project Manager: HCV Treatment, Alliance for Public Health also took part in the Congress as a part of the Ukrainian delegation.
Within the Congress, members of the Ukrainian delegation will take part in a number of meetings with key partners and international stakeholders with a view to expand Alliance programs in Ukraine, strengthen cooperation with partners and develop a plan of further steps.
Recruitment into HCV Treatment Project is Underway

14/04/2016
In March 2016, the first phase of the Project “Scaling up accessible and effective HCV treatment through community-based treatment model for most vulnerable populations in the resource-constrained Ukraine” was successfully competed. Meanwhile, recruitment of patients for the second phase of the project is underway.
Since June 2015, 450 patients representing key populations got access to HCV treatment in 11 healthcare facilities within the first phase of the project, which is 80% more than the planned indicator. Increasing the number of treatment courses available was possible due to the use of pegylated interferon procured within the state budget, which allowed reducing the duration of treatment to 12 weeks and increasing the number of courses.
As of 1 April 2016, 183 new patients received access to hepatitis C virus (hereinafter – HCV) treatment within the second phase of the project, and thus the total number of patients covered with treatment is 633 people.
93% participants of the first phase had HIV, and 94% of them received ART. 80% were people injecting drugs, with 8% of them being OST program clients. 15% of project participants had previous treatment failures when treated with two-component regimen: pegylated interferon + ribavirin. The success of innovative treatment is proved with sustained virological response observed in 90% of patients.
List of criteria for enrolment into the second phase of the project, list of healthcare facilities offering HCV treatment and NGOs providing support to patients may be found here.
Hepatitis C: Game of Survival?!
05/04/2016
On 5 April 2015, Alliance for Public Health (Alliance) together with its partner civil society organizations held Hepatitis C: Game of Survival campaign near the premises of the Cabinet of Ministers of Ukraine. Participants of the campaign demonstrated the “deadly slot machine” to policy-makers and mass media, demanding to solve the urgent problem of allocating budget funding to treat patients with hepatitis C virus (HCV).
According to the official data, there are 73 thousand patients with HCV registered in Ukraine, among them at least 40 thousand urgently need treatment. However, those numbers do not reflect the real situation with the spread of epidemic. As estimated by WHO, more than 5% of people in Ukraine are infected with HCV, which is over 2 million people! It is hard to say how many of them should have already received treatment. There is no official register of patients, though it should be established in accordance with the National Viral Hepatitis Program.
Back in March, people living with HCV, doctors, and civil society organizations were looking for the results of the long-awaited government competitive bidding to procure drugs for HCV treatment, as there was a hope that after international organizations take over the procurement, innovative treatment will become more accessible. However, the bidding results have not been made public yet, and even the expected amount of drugs will cover only a minor share of patients. 6.1 thousand bottles of the modern drug, sofosbuvir, planned to be procured with government funding, may be enough to cover 2,000 standard treatment courses! Such number of patients who will be able to receive treatment will no way help to eliminate the HCV epidemic in Ukraine. According to the best-case scenario, only one of 20 patients who urgently need treatment today, will be able to receive the therapy within the government funding. Patients’ selection criteria should first and foremost depend on the severity of disease, but in practice the existing corruption schemes may make certain adjustments in the selection procedures.
People living with HCV unwillingly became hostages of a very dangerous game, and now their fate depends on the “deadly slot machine” – some of them will be lucky to receive treatment which will give them a chance to survive. Meanwhile, thousands of other patients will not live to see the next round of the slot machine operation! Delays and half words from the side of the Government, the Ministry of Health, and regional administrations make patients and treatment advocates go out on the streets. These days, similar campaigns will also be held in many regions of Ukraine to make responsible public officials review budgets and allocate additional resources to procure treatment courses from local budgets. In 2014-2015, in Ukraine only about 100 patients had a chance to receive treatment at the cost of regional budgets, but unfortunately it is typical when even regions with budget surplus, where over 10 thousand people are registered living with HCV, local authorities count only on the national funding of such treatment. At the same time, there are success stories of regions with budget deficit, which apart from the courses allocated within the National Program, also find a way to procure life-saving treatment within their competence. Most heads of regions count on the funds allocated within the National Program and on the treatment courses provided by Alliance. But such approach is self defeating and extremely ineffective – programs of international donors will be shut down next year, and the National Program will be over in December 2016. Thus we have a paradoxical situation when there is nobody who cares about patients. And the treatment program is available only on paper in order to report on its existence, and even if the program is implemented, it is often done “for the sake of appearance” and not to really save thousands of lives!
“Current HCV treatment is very expensive, in developed countries it may cost up to USD 80,000 per course, – underlines a community activist, Julia Dorokhova. ¬– Today the innovative drugs have already been registered in Ukraine, and hundreds of patients were able to get treatment within the Alliance program. But now we have all found ourselves in a situation when we are players in a “state casino” – nobody knows if any money will be allocated for treatment, if policy-makers will remember about tens of thousands of patients or if they will go on thinking that there is no epidemic of HCV? It is not enough to just approve a program, when people’s lives are at stake”.
At the same time, in the center of Kyiv a mobile clinic was operating, where all people could receive professional counseling on HCV diagnostics and treatment as well as special forms for examinations.
“Not a single country in the world knows the exact number of people infected with hepatitis C virus, because the infection process is hidden, – says Professor Olha Holubovska, Head of Infectious Disease Department at the Bogomolets National Medical University, chief infectious disease specialist of the Ministry of Health of Ukraine. – Our consolidated efforts allowed to approve the National Viral Hepatitis Program as well as 16 regional programs; introduce amendments to the treatment guidelines, which were brought in line with the international standards; and register innovative direct-acting antiviral agents in our country. Now Ukraine has to develop new modern strategies aimed at elimination of viral hepatitis in line with WHO objectives. That is why we are currently working on those tasks together with civil society organizations. Unfortunately, the level of funding of the National Program is very low, with many activities not financed at all. Also, not all regions of the country demonstrate sufficient commitment in this regard – even the existing local programs are either not financed at all or are financed at an extremely low level. To comply with the WHO Global Strategy, it is not enough to provide only treatment – this strategy also stipulates improvement of the epidemiological surveillance system, implementation of prevention activities, etc. If we stop at this stage, further spread of HCV epidemic will have disastrous consequences for Ukraine”.
So far treatment of HCV depends on the operation of the “deadly slot machine”, allowing only one in twenty patients to win a chance to receive life-saving treatment within the state budget. But will this machine operate at all or will the statesmen just forget about tens of thousands of patients waiting for their therapy? The situation with HCV epidemic becomes more and more pressing. The countdown of people’s lives in this Live Casino is going on…
***
Over 150 million people in the world are infected with hepatitis C. The threat of the spread of epidemic in Ukraine grows in the context of the economic crisis and military conflict in Eastern Ukraine. One of the priority areas of the activities of Alliance for Public Health and its partners is response to the epidemic of hepatitis C in Ukraine and provision of HCV diagnostics and treatment for vulnerable groups and general population. In 2015, Alliance launched thefirst program of treatment with a new-generation drug – sofosbuvir, which is expected to cover up to 2,000 people. 203 patients have already completed their treatment courses. As per the set standard, 12 weeks after completion of the therapy patients are to go through a follow-up examination – and it shows that in 90% of those who receive treatment HCV is undetectable. This is a huge step forward as compared to the old treatment regimens (peginterferon + ribavirin), when the indicator of successful treatment outcome was no more than 50%. In 2014, Alliance managed to negotiate significant price reduction with the manufacturer, which allowed for the first time in Ukraine to procure sofosbuvir at the price of USD 900 per course.
Over 5% of Ukrainians are infected with hepatitis C
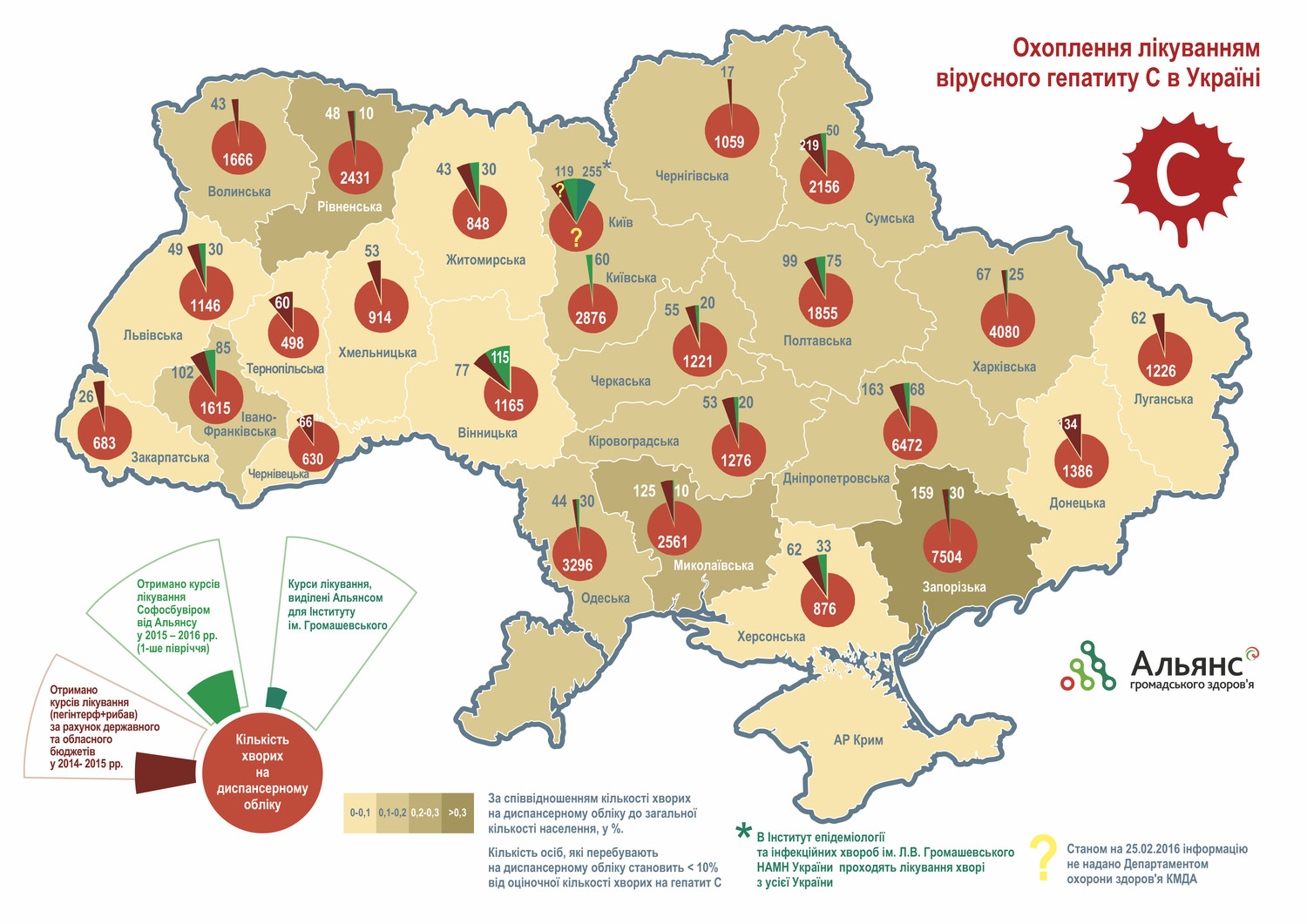
25.02.2016
The results of competitive bidding to procure medical drugs for hepatitis C treatment in adults and children will be announced within the next few days.
For the first time ever, this important function was delegated to the United Nations Development Program (UNDP), which will make innovative treatment of hepatitis C (HCV) more accessible to several thousand patients in Ukraine. It depends on the final price of drugs which will be defined in the contract.
The price for a standard HCV treatment course with original direct – acting antivirals (including sofosbuvir) in developed countries varies from 30 to 80 thousand US dollars, but Alliance for Public Health (hereinafter – Alliance) back in 2015 was able to procure sofosbuvir at the lowest price for the original drug – USD 900 per standard 12-week treatment course. This price became a reference point of the “upper price limit” for the current UNDP tender within 2015 state procurement!
According to the data presented by the World Health Organization (WHO) last year, over 185 million people in the world are infected with HCV, leading to 350 thousand deaths per year. Only in WHO European Region, 84 deaths per year are caused by hepatitis C-related cancer and cirrhosis.
In Ukraine, the epidemic of HCV is growing every year. The main independent infectious disease doctor of the Ministry of Health of Ukraine, Head of theInfectious Disease Department of the Bogomolets National Medical University, Professor Olha Holubovska makes an emphasis on the critical situation with HCV in the country: “According to WHO, in Ukraine over 5% of people are infected with hepatitis C, in absolute numbers it is more than 2 million people. In certain populations, the rate of infections is much higher. Thus, based on the data of the Monitoring and Disease Control Center of theMinistry of Health of Ukraine, the level of infections among people who often stay in hospitals is around 12%, and among patients of drug treatment clinics – up to 67%.”
Back in 2009, Alliance initiated large-scale HCV screening in vulnerable populations, and since 2011 such testing is offered to general population during mass campaigns held within the all-Ukrainian «Demand Treatment!» advocacy campaign. In 2013, the Ukrainian Government approved the first National Targeted Program of HCV Prevention, Diagnostics and Treatment. However, the state allocates funding only for the treatment component, covering only 20% of the existing needs, while other important components of the program, including diagnostics, are not covered from the state budget at all. As a result, we have a vicious cycle: not many people may afford quality diagnostics, which is quite expensive, and as a result the statecannot make a reasonable estimate of the need in HCV treatment, plus it is not really interested in it… due to state budget limitations.
In 2015, Alliance initiated the first program to offer treatment with a new-generation drug – sofosbuvir – which is expected to reach 2,000 patients.According to the results of the first stage of the program, in over 90% out of several dozens of patients who already received treatment HCV was not detected! Based on our treatment experience, at the end of the previous year amendments were introduced to the Unified Clinical Guidelines “ViralHepatitis C”, and the drug was included into the State Register of Medical Drugs and the national list of drugs which may be procured with budget funds. It will allow to provide treatment to hundreds thousands of patients.
“Based on the practical experience of Alliance in expanding access to HCV treatment, we found that civil society and international organizations canmake an important contribution to the response to the epidemic. For this purpose, we consistently join our efforts with all stakeholders, – underlines Ludmila Maistat, Senior Program Manager: Hepatitis, Alliance for Public Health. – We anticipate that at the next session of the World Health Assemblymember states will approve the first Global Health Sector Strategy for Viral Hepatitis, into the development of which Alliance has also contributed.Provided that there is sufficient funding, reduced prices for direct-acting antivirals, and access to quality generic drugs, Ukraine has a good chance to successfully implement this strategy and radically scale up access to innovative HCV treatment.”
Due to the critical underfunding of the national hepatitis program, the drugs which are planned to be procured with state budget funds will cover thereal needs only in part. Alliance was able to launch treatment with support of the international donors, but the amounts and the timeframes of suchfunding are also limited.
Alliance implements its programs in close cooperation with the Ministry of Health of Ukraine, having developed a joint vision of eliminating theepidemic in the country – including only modern drugs into treatment regimens, in particular quality generics, holding further negotiations withpharmaceutical companies, reducing prices for diagnostics and expanding the network of diagnostic centers, training of doctors and patients, and making a special focus on most vulnerable populations, with large-scale preventive activities among the general population – those are the comprehensive measures which will help to eliminate the HCV epidemic!
The experience of Georgia may be used as an example of efficient approach in the response to HCV epidemic. Government of the home country ofcurrent Ukrainian Minister of Health covers 70% of the cost of HCV treatment in most disadvantaged populations, with the remaining 30% provided by the municipal budgets. For other categories of patients, the state compensates 30% of the cost of treatment, with the remaining 70% allocated between the local budget and the patient, depending on the income of the latter. Such funding scheme functions like a certain social guarantee for low-income citizens. Free treatment of the members of vulnerable populations was defined as a paramount and top-priority task as this approach, according to WHO recommendations, is one of the key factors in making an impact on the epidemic. In Georgia, where the population is much smaller than inUkraine, the Government reached an agreement with the manufacturer on allocation of 20,000 HCV treatment courses and financial donations to improve the system of patient registration and effective medical support of the project.
The results of HCV screening held among over 4thousands of those conscripted to the military forces, those involved into the military operations in theEast of Ukraine and demobilized soldiers, held by Alliance in 2015, demonstrated a rather high level of infections – about 4%, and in the main military hospitals – even up to 10%, which made the Ministry of Defense of Ukraine and mass media finally turned their attention to this burning issue.
In the time of military conflict in the East of our country and long-term economic crisis, we need to join our efforts in response to the epidemic. Sharp reduction in the cost of diagnostics and treatment with advanced drugs, their procurement at adequate prices (taking into account the price already achieved by Alliance in 2015) will become crucial and prominent steps on this way, which will prove the commitment of the state to care about the health of its citizens and will allow saving thousands of lives even in 2016!
Successful results of Sofosbuvir-Based Treatment of HepC!

It has not been long since Ukraine first started recognizing sofosbuvir as a highly effective drug for the treatment of hepatitis C virus (HCV); and only several months ago sofosbuvir was included into the National Drug Register and finally was listed in the tenders for the procurement of medicines within the state budget, which are first carried out through international organizations (in particular though the United Nations Development Programme (UNDP)).
While the state sector is only getting prepared for the long-awaited treatment with sofosbuvir, the Alliance for Public Health (hereinafter–Alliance) reports on the first successful results of treatment with this innovative drug.
In 2015, Alliance was the first in Ukraine to launch the program of sofosbuvir-based HCV treatment for 1,500 members of vulnerable populations, mostly for people with HIV/HCV co-infection and people who use drugs. Thanks to the cooperation with the Ministry of Health and support of international donors, as of 1 February 2016, 379 patients, including 366 those with HIV/HCV co-infection, already accessed the effective treatment.
203 patients have already completed their treatment courses! As per the set standard, 12 weeks after completion of the therapy patients are to go through a follow-up examination – and it shows that in 90% of those who receive treatment HCV is undetectable. This is a huge step forward as compared to the old treatment regimens (peginterferon + ribavirin), when the indicator of successful treatment outcome was no more than 50%. After sofosbuvir proved to be effective in the pilot HCV treatment program implemented by Alliance, in 2015 the drug was included into the Unified Clinical HCV Treatment Guidelines.
Within the Alliance treatment program, Ukrainian doctors have a chance to consult with international experts and participate in trainings on the innovative international practices of hepatitis treatment. As for the patients, they get not only drugs, but also social support of non-governmental organizations, which allows ensuring higher adherence to treatment and increases the probability of successful treatment outcome.
In January 2016, the second phase of Alliance HCV treatment program started, which will cover at least 500 patients. We hope that the recently announced tender for sofosbuvir procurement with state budget funds through international organizations (UNDP) will be held in a transparent and effective manner, and the price for the drug will not exceed Alliance procurement price for 2015! In 2014, Alliance managed to negotiate significant price reduction with the manufacturer, which allowed for the first time in Ukraine to procure sofosbuvir at the price of USD 900 per course (in some countries the price for such treatment course may exceed USD 80,000 (!)). It should be noted that it were the activities initiated by Alliance, such as the all-Ukrainian “Demand Treatment!” advocacy campaign and the innovative treatment program, which encouraged not only concerned civil society advocates but also public officials and doctors to talk about the HCV epidemic, with the National Targeted HCV Treatment Program finally approved.
We realize that it is only the beginning. The patients who receive or are expected to start the therapy represent only a small share of patients who urgently need treatment. But the first steps have been taken, and it gives hope to thousands of patients with hepatitis C in Ukraine. The Alliance for Public Health continues systematic efforts to advocate for the interests of people who are in need of life-saving treatment both at the national and international levels.
Contact person: Myroslava Andruschenko 095 271 09 31, andrushchenko@aph.org.ua
Alliance Reinforces the Regional Partnership in Hepatitis C Treatment

04/02/2016
Within the efforts aimed at expanding the access to hepatitis C (HCV) treatment in vulnerable populations, Alliance for Public Health holds a working meeting and a training for 70 healthcare professionals and representatives of NGOs from 15 oblasts of Ukraine, involved into implementation of the second phase of Alliance DAA-based HCV treatment program. In January 2016, a new phase of program implementation commenced, allowing 500 more patients to access effective HCV treatment.
On 3 February 2016, members of the working meeting in Kyiv, including representatives of the Ministry of Health, the Ukrainian Center for Socially Dangerous Diseases Control, chief doctors of healthcare institutions, NGO managers, doctors, civil society activists, and patients with HCV, discuss the ways to reinforce partnership and improve the efficiency of HCV treatment in Ukraine within the second phase of Alliance treatment program.
The training for healthcare professionals and social workers of NGOs on 4-5 February 2016 will include presentation of theoretical materials and development of medical and social support skills in HCV treatment in vulnerable populations using multidisciplinary approach, prevention of re-infections, use of innovative drugs in HCV treatment, and treatment monitoring.
Expanding Access to Hepatitis C Treatment

03.02.2016
Alliance expands access to hepatitis C treatment for vulnerable populations in Ukraine.
Starting from January 2016, Alliance for Public Health launched the second phase of program for vulnerable populations, which stipulates treatment of hepatitis C virus (HCV) with innovative direct-acting antiviral agent, Sofosbuvir.
Thanks to the National Campaign “Demand Treatment!” initiated by Alliance and persistent advocacy activities in cooperation with partners from all regions of Ukraine, in October 2013, for the first time in Ukraine, Peginterferon and Ribavirin-based HCV treatment program was launched, and since2015, also for the first time in the country, Alliance initiated treatment with Sofosbuvir. As of 1 January 2016, there were 320 patients involved into the program, with 161 of them having already completed their treatment courses (mostly all of them demonstrated undetectable HCV at their final tests). Before the end of the year, at least 1,500 patients will have access to such treatment. Advocacy efforts of Alliance as well as confirmed treatment success of Sofosbuvir allowed including it to the State Register of Medicines and the Unified HCV Treatment Guidelines, opening the way for government procurement and inclusion of the drug into the state-funded treatment programs.
This year, HCV treatment will become available in five more regions of Ukraine (the total of 14 oblasts will be reached), with planned coverage of at least 500 patients. The list of healthcare facilities which offer treatment and NGOs providing support to patients is available here.
According to the updated criteria of patient selection, the treatment will be accessible not only to people with HIV/HCV co-infection, but also to members of other vulnerable populations, in particular opioid substitution treatment patients, people who inject drugs, commercial sex workers, etc. with no HIV.
Important notices for the patients who comply with selection criteria:
• In oblasts, which offer treatment only in AIDS centers, such treatment will be accessible only to people with HIV.
• Alliance, supporting I Speak Out Now! anti-corruption campaign of the Global Fund (http://www.ispeakoutnow.org/home-page-ru/), draws attention to the fact that one of the priorities of HCV treatment program is protection of the rights of patients from among vulnerable populations, in particular their right to free treatment. If any corruption situations occur when enrolling patients into the program or if there are any unreasonable proposals from the project staff to receive illegal rewards from patients, please write the details of such situations to Alliance e-mail porada@aph.org.ua.
Alliance does not rest on its oars, but continues its advocacy and treatment efforts to make an impact in response to the HCV epidemic in Ukraine.

