Study: Community based HIV treatment for IDUs in Ukraine (‘CITI-Plus’ study)
Study: Community-based HIV treatment for people who inject drugs in Ukraine (CITI-Plus study)
The ‘CITI-Plus’ study compares 1,350 PWID on ART at CITI sites, CITI-Plus sites and Standard-of-Care (SoC) sites. Participants are interviewed at baseline with follow up at 6, 12, and 18 months. Information on HIV care enrollment, ART initiation, retention, clinical outcomes (CD4 counts, viral loads, morbidity and mortality) is collected.
Study objectives and design
This project evaluates the effectiveness and efficiency of CITI and CITI-Plus interventions.
The goal is to assess CITI’s influence on treatment start, adherence and retention, and if additional components of community-based contingency management (CCM) have any effect on study outcomes.
More specifically, the project will introduce adherence focused interventions such as CCM, to improve adherence among PWID accessing HIV treatment through community initiated treatment intervention (CITI – previously known as the HIV peer-navigation project, a pilot project supported from March 2013 within the Global Fund grant).
All CCM and CITI approaches are built on the meaningful personal links most HIV-positive people have. This is an innovation compared to conventional approaches where case management, adherence counseling and support are provided externally through staff of health care organizations and clinics.
Main research questions
- Do the CITI and the CITI Plus (CCM) interventions improve access, adherence and retention to ART and clinical outcomes of HIV-positive PWID in Ukraine?
- What are the bottlenecks in PWID’s access to ART in Ukraine?
Research Team: Roles and Responsibilities
The study is a collaboration between the following partners:
/1
International HIV/AIDS Alliance (UK) providing general project coordination
/2
Alliance for Public Health. As a main partner the Alliance will be responsible for appropriate site staff training and support intervention and protocol compliance at intervention sites. Other competencies include training of scientific staff, participation in data analysis and results dissemination at local level.
/3
US scientific agencies (Institute of National Development, scientific institutions, others) will lead in protocols elaboration, data analysis and report writing. This partner will also be responsible for scientific mentoring and capacity building, staff training and quality assurance studies.
Study Design:
the proposed study aims will be achieved using quasi-experimental design.
The study is designed to assess the effect of three interventions – Standard of Care(SoC), Community Initiated Treatment Intervention (CITI) and CITI Plus – on HIV care engagement, ART enrollment and retention, and on clinical outcomes (CD4 counts, viral loads, morbidity and mortality).
We plan to measure the progress of HIV-positive PWID along the cascade of services related to HIV treatment, i.e. initiation of treatment and treatment adherence and retention in six experimental sites (six cities of Ukraine) with combinations of CITI, and in six control sites with no community-based interventions related to HIV treatment. The six control sites will offer only the standard AIDS center service package in HIV treatment, care and support.
General overview
There are experimental sites in nine cities (Poltava, Kharkiv, Mykolaiv, Dnipropetrovsk, Lviv, Odesa, Kiev, Nikopol, Apostolovo), five with CITI and four with CITI Plus intervention.
Control sites are presented by locations where no CITI or CITI Plus projects are implemented and only a basic package of harm reduction (HR) services for PWID is present (SoC locations). Six control sites are in five cities (Sumy, Chernihiv, Khmelnytsky, Zhytomyr, Vinnytsia) with no community-based interventions related to HIV treatment (SoC).
On the basis of collected results, statistical analysis will evaluate associations between the study’s outcome measures, and potential determinants. Outcome measures are defined as:
- Adherence to ART
- ART Retention
- Level of drop-outs from treatment
- Time needed to access treatment (official registration at local AIDS clinics, ART start)
- CD4 counts
- Viral loads
- Morbidity and mortality
Potential determinants of the study are:
- Involvement in SoC
- Involvement in CITI
- Involvement in CITI Plus (CCM)
- Experience of VCT in HR
- Previous history of HIV treatment (including drop-outs)
- Structural and individual barriers to access ART
- History of incarceration and arrest
- Drug use behavior
- Sexual behavior (condom uptake, number of sexual partners, involvement in commercial sex work)
The covariates are defined as:
- Age
- Gender
- City (region of Ukraine)
- Family status (children)
- Main occupation
- Level of income (permanent or temporary)
- Place of living
- Educational level
- Sexual orientation
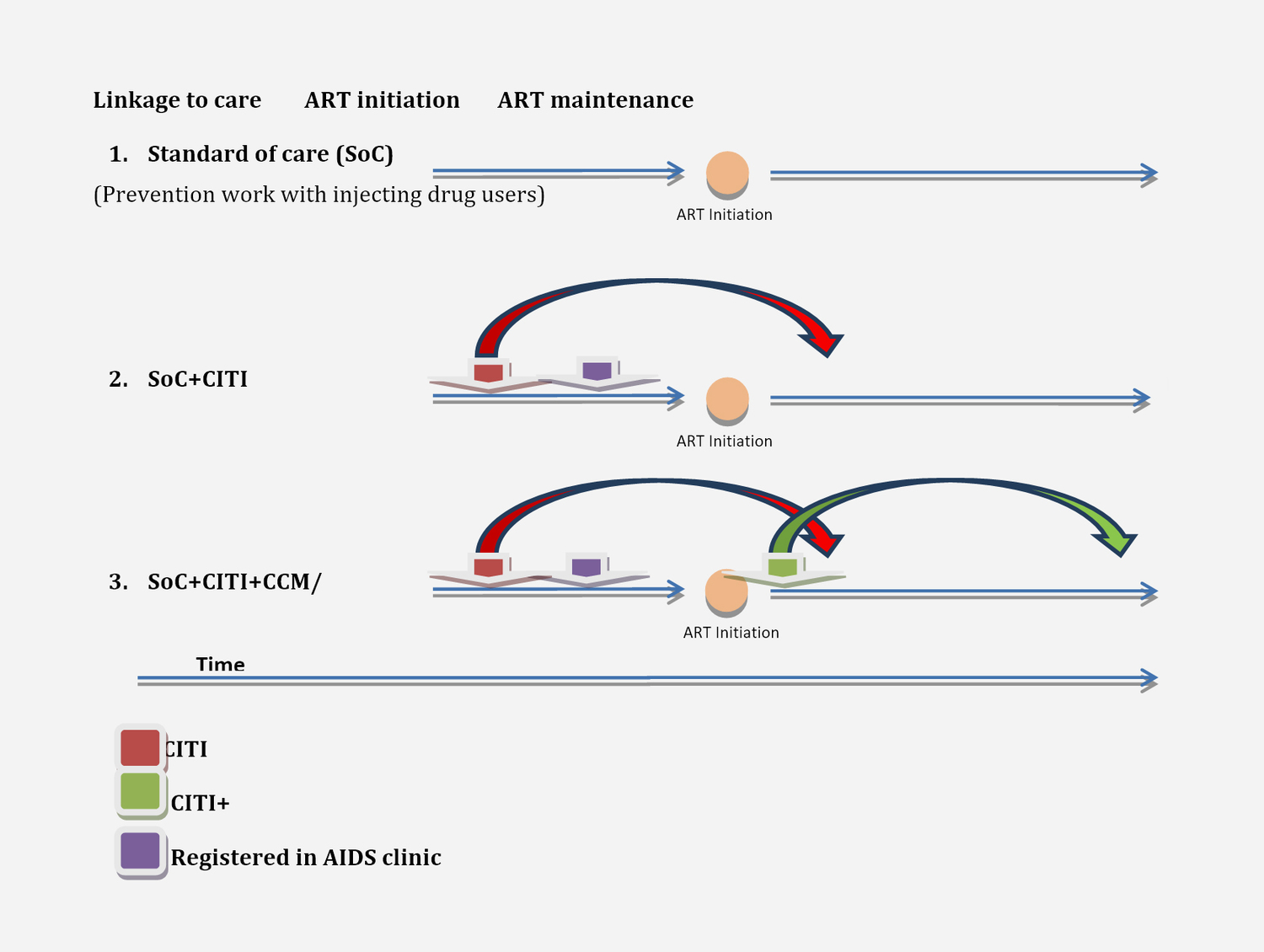
Figure 1. Study design scenarios
Contact person: Denisiuk Olga

